Aseptic Filling
Optima Pharma specializes in aseptic filling for the pharmaceutical industry. Providing top-notch solutions that meet the highest standards and address the unique needs of the pharmaceutical and biotech industries. Their goal is to create advanced technology that keeps every product handled are safe, sterile and of excellent quality.
Optima Pharma are among the best manufacturers in the business. Their integrated solutions cover everything from advanced aseptic filling machines to complete pharmaceutical production lines. They work every day to help you produce more and better, and are dedicated to making your aseptic filling processes as efficient as possible.
Optima Pharma is the “home of turnkey” , one of the few providers on the market to offer comprehensive turnkey solutions. Thanks to the innovative CSPE approach (Comprehensive Scientific Process Engineering), which uses digital twins and detailed simulations, they can seamlessly integrate filling lines with isolator and lyophilizer (freeze-dryer). With this pre-emptive synergy, can be sure that all elements of the sterile environment will work together perfectly and won’t have to worry about any nasty surprises on site when assembling large units.
Often encounter that time and budget, challenges with filling and packaging high-quality pharmaceuticals in vials and infusion bottles. With proper input, we will design the ideal solution for your requirements. The time-to-market must be short and product losses minimal. We repeatedly demonstrate our expertise as a turnkey partner in system design with the successful implementation of complex vial systems, complete with isolators and freeze-dryers.
Optima utilizes the Comprehensive Scientific Process Engineering (CSPE) approach to ensure that the product gets to market quickly and safely. During operation, smart innovations minimize process and system downtime, as well as reducing product losses to a maximum of one object per batch. Digital components and services also guarantee uncompromising quality, maximum safety and optimal flexibility.um safety and optimal flexibility.
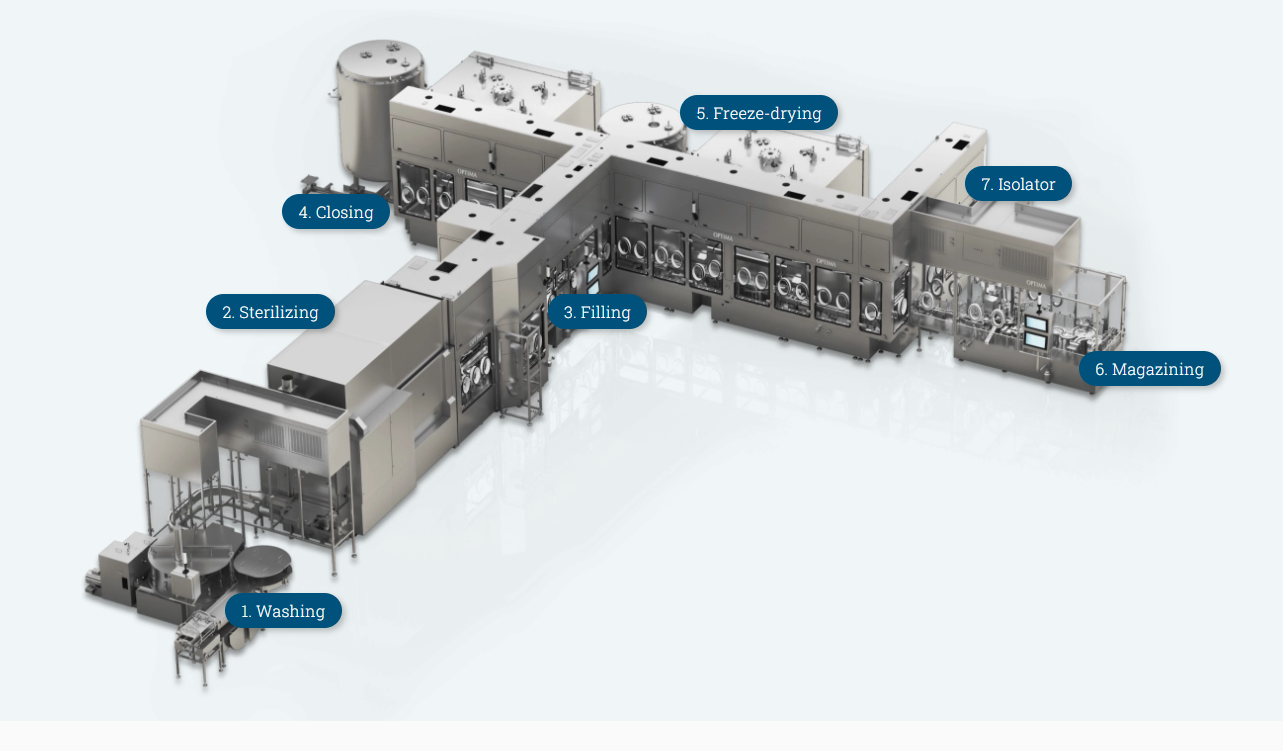
- Washing
The first step is to clean the vials and infusion bottles in fully automated washing machines – available with optional ultrasonic pre-treatment. Single or multi-point grippers transport the products in either intermittent or continuous motion. Special designs can reduce the water consumption by up to 45%.
Benefits:
- Effective and thorough cleaning
- Intermittent or continuous motion
- Reduced water consumption
- Sterilizing
After the glass containers progress through the washer, they proceed to the sterilization tunnel where they are sterilized and dehydrogenized. From cleaning and sterilization to sterile filling, all Optima systems are part of a complete aseptic filling line that provide a continuous process in cleanroom conditions.
Benefits:
- Sterilization tunnel for every application
- Gentle heating of the objects
- End-to-end process under cleanroom conditions
- Filling
Whether viscous or water-like liquids, Optima offer the appropriate filling solution for every product type and batch size. If the right solution isn’t already available, Optima will work with the client to develop it. Our experience with a wide range of applications, like biotech, biosimilar, generic medicines, vaccines, blood plasma or oncology give us unique knowledge to create a solution for your needs. Available filling technologies include rotary piston pumps, peristaltic pumps or time pressure filling systems. Additionally, other filling systems can also be integrated.
Benefits:
- Depending on the product: Vacuum or sensor filling, gassing etc.
- 100% in-process control as standard
- Filling path optional with single-use or CIP/SIP
- Integrity tests such as PUPSIT are available
- Safe rake transport, quick format change
- Closing
Systems close the vials and infusion bottles fully automatically with all standard closures using state-of-the-art closing machines. These machines handle all standard closures for closed vials, ensuring a secure vial cap every time. Optima employs technology that minimizes product loss to maximize yield. Advanced techniques such as gassing during the closing process ensure a residual oxygen content of less than one percent, which is crucial when filling highly sensitive products.
Benefits:
- Compact design
- Fully automated closing (stoppering and capping)
- Compatible with all standard closures
- Minimal product loss
- Freeze-drying with filling and unloading
The fifth step, if required by the product, is lyophilization or freeze-drying, extending the shelf life of the ingredients. As a turnkey provider, connect this step seamlessly to the filling line – for optimal efficiency in your production process. The freeze-dryers are scalable for every production quantity, from small clinical batches to large commercial batches. Optima also offers loading and unloading systems tailored to your freeze-drying requirements – from manual to fully automatic systems.
- Magazining
At the end of the process chain, the system collects the objects in a magazine. The injection and infusion bottles are magazined by the system in the exact quantities and hexagonally offset. The magazining system can fill two or four magazines alternately. This means that magazine changes take place without stopping the machine.
Benefits:
- Magazining in exact quantities
- Magazining with hexagonal offset
- Magazine change without stopping the machine
- Isolator
Containment solutions such as isolators supplement the turnkey portfolio for vial and bottle filling. The isolators can be combined with any type of pharmaceutical system.
Syringes, cartridges or vials : Filling and packaging pre-sterilized components in ready-to-use (RTU) containers requires specialized system technology. This is Optima’s core competence: From custom-fit syringe filling machines to complete turnkey solutions, including pharmaceutical isolators for your application.
Time and cost in pharmaceutical developments are increasing. To start with the production, feeling more confident and, above all, faster, Optima have developed CSPE (Comprehensive Scientific Process Engineering) and other intelligent end-to-end processes.
Optima design system technology for your primary packaging and secondary processing, which are agile and precisely tailored to your needs, based on Optima’s technology platforms. Encompass all steps from filling and closing, including upstream and downstream processes: From nest removal to labelling, as well as the insertion of piston rods, assembly of safety devices, finger flanges and much more.
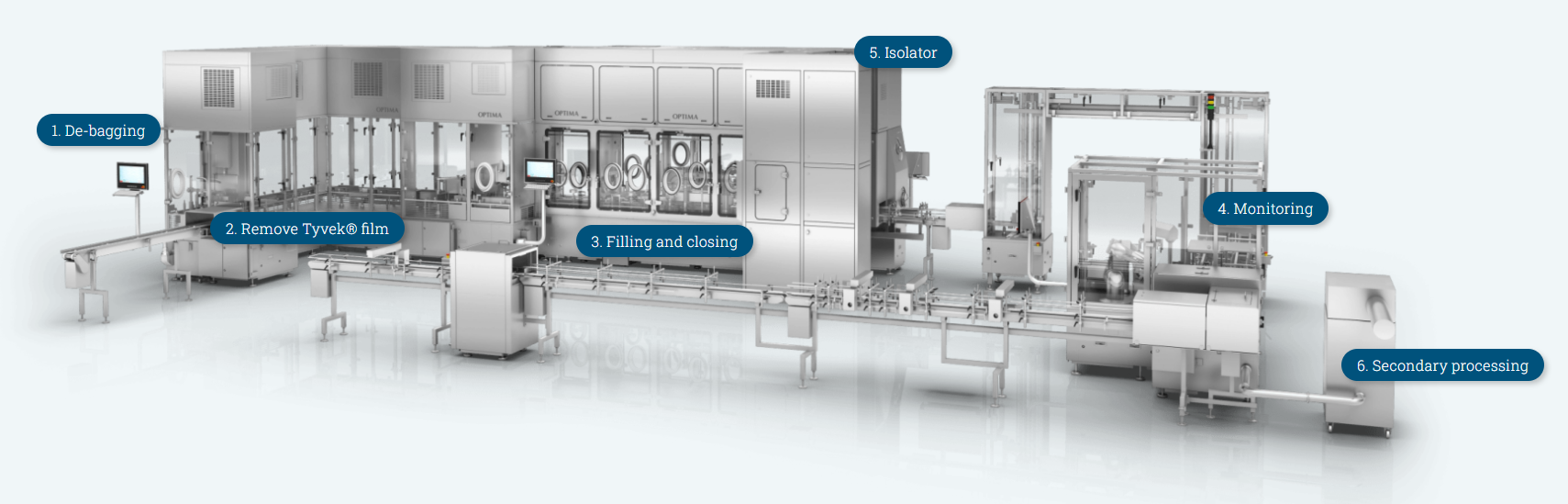
- De-bagging
Whether the RTU packaging materials arrive single or double bagged at the filling line, you can choose a manual, semi-automatic or fully automatic system from our portfolio. Bags are opened to remove the tubs. The tubs are pushed out of the bag, without being touched and then transferred to the sterile area.
Benefits:
- Unpacking of the tubs either manually, semi-automatically or fully automatically
- The tub is transported, to the next station, without being touched
- Remove Tyvek® film
A robot specifically designed for use in the cleanroom removes the intermediate layer of Tyvek® film.
Benefits:
- Sterile room compatible robot system
- Clear separation of cleanroom areas
- Compact and pharmaceutical grade design
- Filling and closing
Whether viscous or fluid: With the correct aseptic filling system, can accurately fill any type of product – with either rotary piston pumps, peristaltic pumps or a time-pressure filling system. Solutions like single-use or CIP/SIP (cleaning-in-place/sterilization-in-place) for the filling path are available. Upon request, containers can be filled and closed under vacuum.
No matter how the system is envisioned, together will develop the optimal solution. No matter what’s selected, the design is compact, easily accessible and easy to operate.
Benefits:
- Filling and closing of nested prefilled syringes, vials and cartridges
- Up to 60,000 pieces per hour
- Filling systems and filling paths to accommodate the product
- Application-friendly systems with integrated in-process control
- Monitoring
Short acronym, big effect: The SIRM (Syringe Inspection and Reject Machine) is an exceptionally safe inspection system for syringes, a crucial component of our syringe filling equipment. It uses a robot and several cameras to remove defective syringes or cartridges from the tub. It also verifies the stopper height of the objects.
Benefits:
- Automated inspection of syringes or cartridges
- Discharge of individual objects
- Maximum security
- Isolator
Containment solutions including isolators complement Optima’s turnkey portfolio for filling RTU nested containers. These isolators can be combined with any type of pharmaceutical equipment, as well as numerous process functions.
- Secondary processing
For process filled and closed RTU containers, choose from the comprehensive machine portfolio for secondary processing: Solutions for labelling, inserting and assembling piston rods, backstops and safety devices. Syringe transport systems, buffer systems, denesters and renesters complete the syringe secondary-processing portfolio.
Benefits:
- Large portfolio for secondary processing
- Compact systems for labelling and inserting piston rods
- Appropriate backstop and safety device assembly machines
- Syringes and buffer systems to meet your space requirements
- Everything from one manufacturer: Saving time and reducing interfaces
Optima have launched OPTIMA MultiUse to give total flexibility for all filling needs. The system profile covers all applications from product and process development in R&D to clinical trials and commercial production – quickly and economically.
The pharmaceuticals market is diverse and fast-paced and production will stay on track with MultiUse systems from Optima Pharma: can process different batch sizes, packaging and containers flexibly and cost-effectively. The system covers all performance requirements. It is designed to handle different product characteristics including liquid, viscous and freeze-dried products.
As a turnkey partner, all process steps upon request are integrated, including isolators and freeze dryers. And with the CSPE process, the MultiUse system up and running in record time. CSPE means „Comprehensive Scientific Process Engineering“ and it speeds up the system’s development and assembly. Select total flexibility for the sterile filling process – with the Optima MultiUse system.
Many reasons – one partner (Benefits):
- Multiple packaging materials and containers –
Filling syringes, vials, and cartridges into nests, trays, or bulk. - Multiple products and active ingredients –
Can process products from liquid to high viscosity and high potency. - Maximum flexibility –
Fast format changes, smart use of robotic systems. - Minimal product loss
With smart technologies and in-process controls. - Turnkey integration –
Isolator, Ready-To-Use (RTU), Bulk and Lyo path are easily integrated. - Absolute safety –
Wide range of inspection and in-process-controls, no glass-to-glass-contact
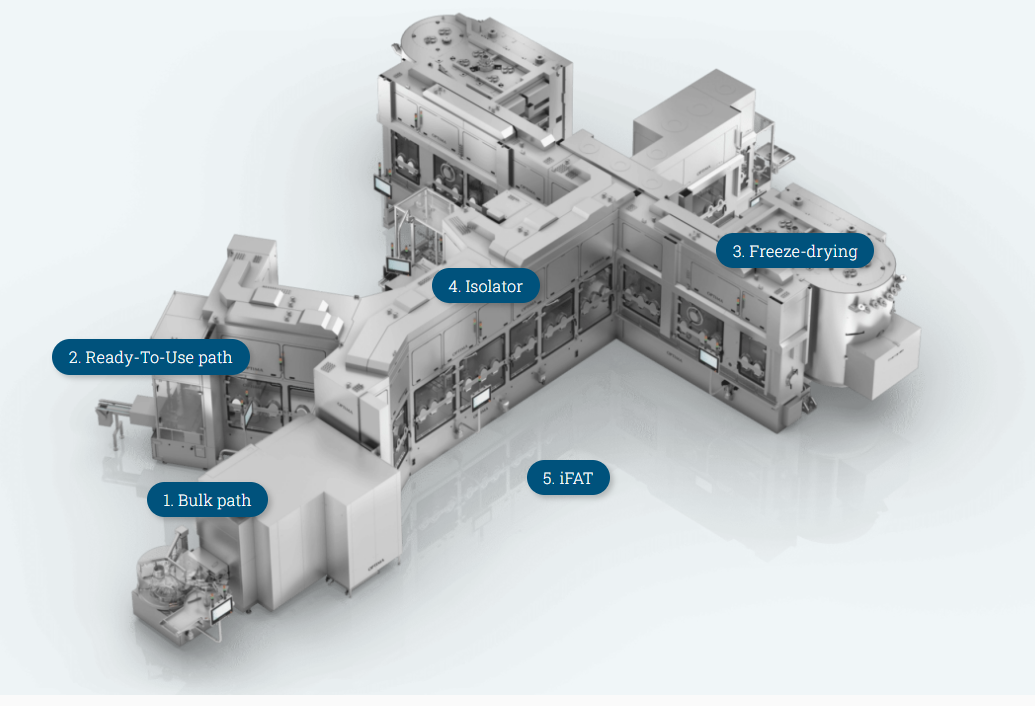
- Bulk path
Vials that are not pre-sterilized arrive at the dosing station from the washing machine and the sterilization tunnel.
- Ready-To-Use path
Pre-sterilized syringes, cartridges and vials in nests or trays arrive at the same filling station using the RTU path.
- Freeze-drying
Can be integrated and completed with a crimping station.
- Isolator
Optional for all process steps.
- iFAT
In order to perform the integrated Factory Acceptance Test, assemble the entire system on Optima premises and perform cycle development. This ensures a safe production start in record time.